Quality, Regulatory, Clinical, and Commercial Software for Emerging Biotechs
Standardized, pre-configured Veeva applications without the implementation and maintenance costs.
Veeva Basics
Industry-leading Veeva Vault with zero implementation
Best of breed Veeva applications now pre-configured and pre-validated to be up and running quickly and easily.

Live in Weeks, Not Months
Your system will be ready to use on day 1. Veeva will guide you through the following 30-60 days to be ready for the first business users.
Always Current
Veeva-managed updates, delivered three times a year, to keep your Vault current and following industry best practices. Benefit from the latest features, with minimal impact.
Pre-Validated
Access complete validation packages on-demand. Every release is validated by Veeva. Conduct self-service vendor audits instantly.
Global Support & Training
End-user 24x7 support provided by the Veeva Global Support Center. Robust on-demand user training for each application available just a click away.
Graduate to Full Vault
Be ready to scale. Veeva Basics uses the same Vault Platform used across the life sciences industry. Graduate to full Vault, no migration required.
Veeva Quality
QualityDocs, Training, QMS, and LIMS
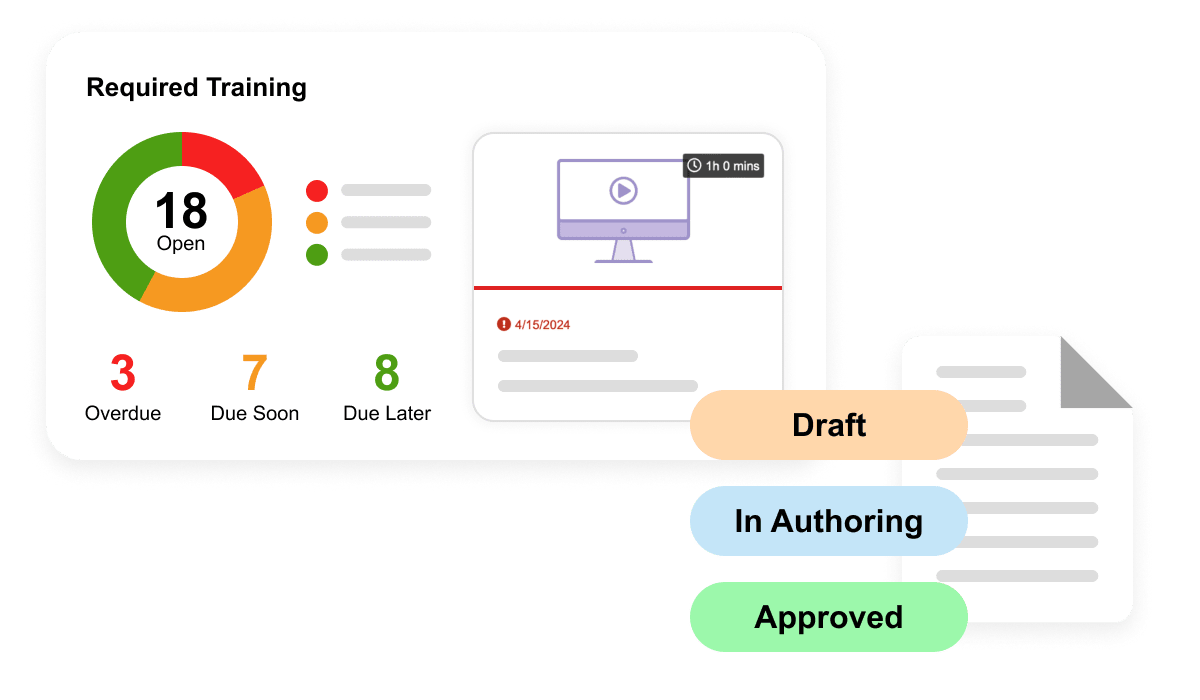
Veeva QualityDocs Basics is the industry-leading regulated quality content management solution. Including best practices for managing SOPs and other regulated content.
Veeva Training Basics is an industry-specific LMS ensuring that your team is ready to perform their jobs while enabling GxP compliance and audit readiness.
Veeva QMS Basics enables seamless management and tracking of quality processes. Includes Audits, Change Control, Deviations, and Standalone CAPA.
Veeva LIMS Basics empowers virtual life science companies to digitize and scale their QC data. It centralizes specifications and external testing results for consistent data, compliance, and accelerated batch release.
LearnGxP is a specialized, industry-focused library of accredited GxP training, providing a seamless way to train your team and maintain ongoing compliance.
Browse our catalog of training content that is relevant to emerging biotech companies.
Veeva RIM
Submissions, Submissions Archive, and Submissions Publishing
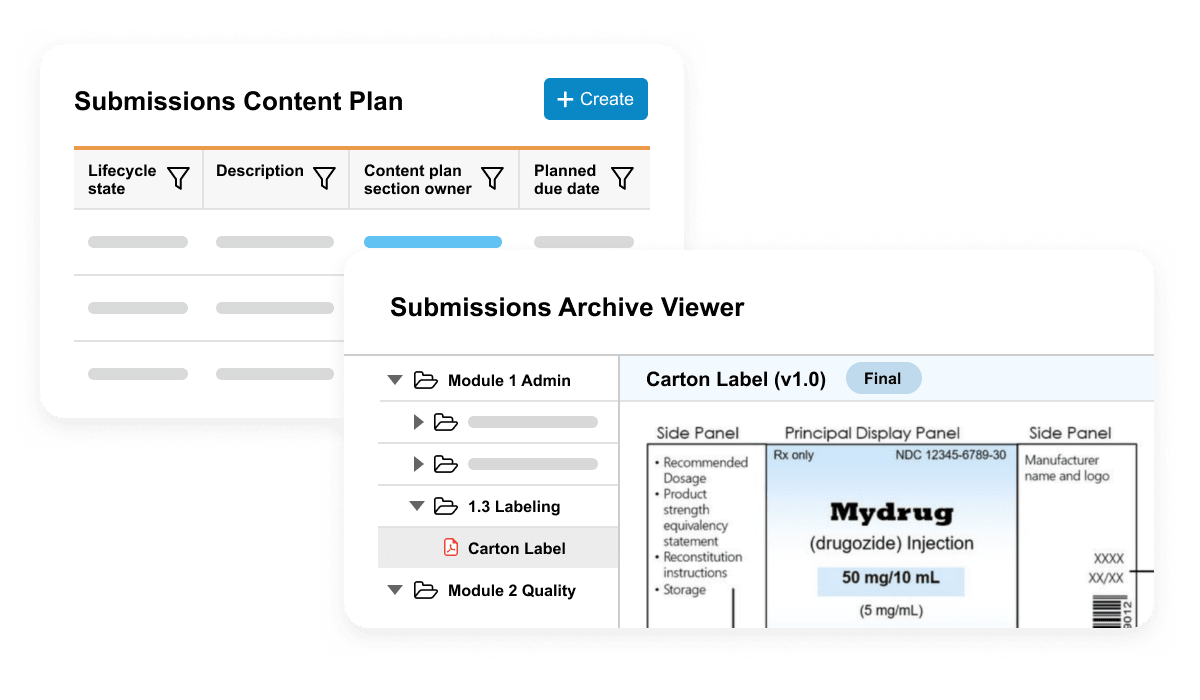
Veeva Submissions Basics is used to plan, collaboratively author, review, and approve regulatory documents. It includes dashboards and reports to allow submission managers to track the status of documents in real time.
Veeva Submissions Archive Basics is a secure repository of submission-published output and includes a viewer for easy access to historical applications submitted to health authorities.
Veeva Submissions Publishing Basics streamlines the assembly, formatting, and publishing of regulatory submissions, ensuring compliance with health authority requirements.
Veeva Clinical
eTMF and CTMS

Veeva eTMF Basics is the leading trial master file application used to ensure quality, timeliness, and completeness of a TMF. It provides full content management capabilities for upload, version control, QC/approval, and real-time co-authoring for study documents.
Veeva CTMS Basics allows for more complete management of your trials including Study Milestones, Monitoring, Subject Enrollment, Issue Management, and Site Communication Tracking.
Veeva Commercial
PromoMats
.svg)
Veeva PromoMats Basics provides the essential end-to-end solution for MLR, Claims, and Digital Asset Management (DAM), guaranteeing intuitive content management and compliant approval of all your promotional content.
Powered by Veeva Vault
Built for Emerging Biotech, Managed
and Maintained by Veeva